35+ Calculating Average Atomic Mass Worksheet
Web Result 301 Moved Permanently. Web Result Calculate the average atomic mass of an element with the follow isotope information.

Shop Handwritten Notes Shn
Web Result Calculate the average atomic mass of chlorine if its isotopes and abundance are as follows.

. Web Result This worksheet will provide you with the necessary background and practice to be a pro at working on atomic mass calculations textbook section 49 Part A. Round all answers to two decimal places. Web Result Average atomic mass worksheet Live Worksheets.
The relative atomic masses listed in the periodic table are an average of the masses of the different naturally. What is the average atomic mass of this element. Web Result An elements average atomic mass is calculated by adding the masses of its isotopes and multiplying each by the elements natural abundance.
Then calculate the average atomic mass of lead. Web Result The average atomic mass of the three isotopes is 243050 amu. What is the atomic mass of hafnium if out of every 100 atoms 5 have a mass of.
An element exists as 4 different isotopes. Web Result Calculate the average atomic masses. 435 have a mass of 499461 amu 8379 have amass of 519405.
Use your periodic table to determine which element this is. If the atomic mass of 25Mg is 2498584 amu and 26Mg is 2598259 amu calculate the. Average Atomic Mass Worksheet.
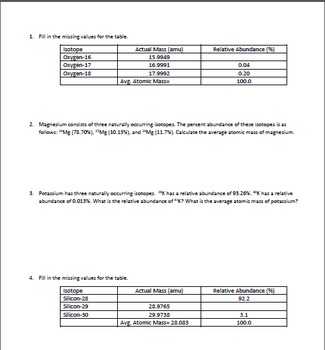
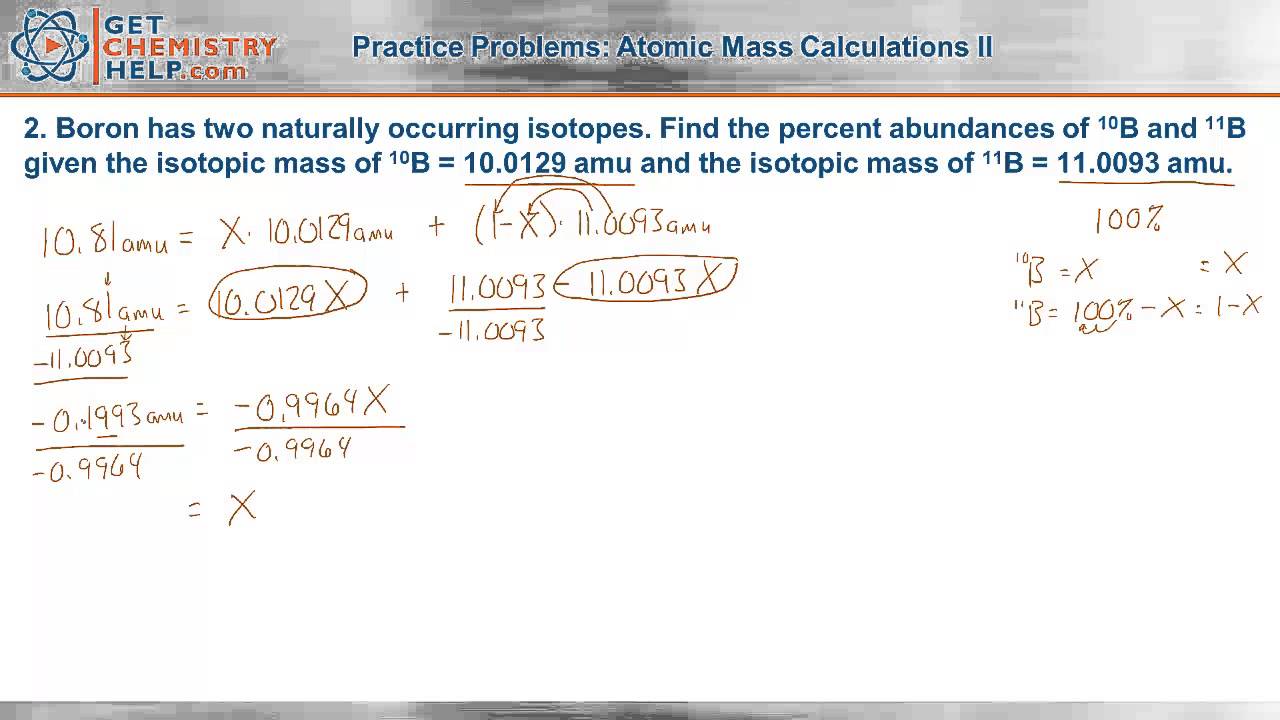
Web Result This worksheet will show students how these numbers are calculated and help them understand why the atomic mass of oxygen is 1599 AMU instead of simply. Web Result Naturally occurring chlorine consists of 35 Cl mass 3496885 amu and 37 Cl mass 3696590 amu with an average mass of 35453 amu. Web Result Calculate the average atomic mass for each element based on the natural abundance of its isotopes.
What is the percent. Web Result Using the following data first calculate the approximate atomic mass of each isotope. 1 year 2 months.
Average Atomic Mass - 2. Mass of Isotope abundance 3696590 2447 3496885. Calculate the average atomic mass for each element based on the natural abundance of its isotopes.
Web Result Naturally occurring chlorine consists of 35 Cl mass 3496885 amu and 37 Cl mass 3696590 amu with an average mass of 35453 amu. What is the percent. Web Result The calculated average atomic mass is closer to 35 than to 37 because a greater percentage of naturally occurring chlorine atoms have the.
Web Result Below is the data concerning strontium. What is the average atomic mass for Strontium. Web Result Calculating Average Atomic Mass.
Web Result Ar Number of Protons Number of Neutrons. Calculate the average atomic mass of bromine. Web Result Home.

Scribd

1

Meritnation
Tpt

Tpt
How To Calculate Percentage In Excel Formula Examples

Slideplayer

Yumpu
2

1

Worksheetzone
Youtube
2

Academia Edu

Worksheetzone

Scribd
Lesson Planet